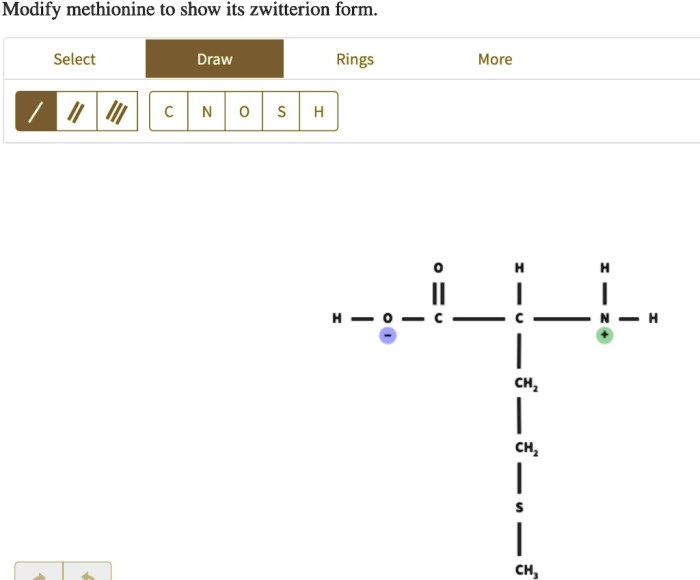
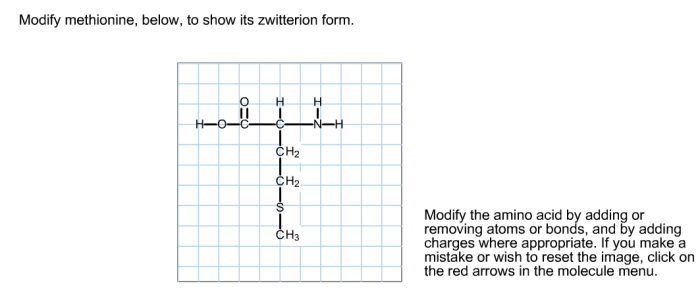
Modify methionine to show its zwitterion form. – Methionine, an essential amino acid, unveils its zwitterionic nature under specific conditions, offering insights into its unique properties and applications. By modifying methionine, we can showcase its zwitterion form, revealing its biological significance and potential.
This comprehensive guide explores the structural formula of methionine, the concept of zwitterions, and the factors influencing zwitterion formation. We delve into the applications of methionine zwitterions, uncovering their biological and industrial relevance.
Structural Formula of Methionine
Methionine is an essential amino acid with the chemical formula CH 3SCH 2CH 2CH(NH 2)COOH. Its structural formula can be represented as:
- Thiol (-SH) group
- Carboxyl (-COOH) group
- Amino (-NH 2) group
Zwitterion Formation in Methionine: Modify Methionine To Show Its Zwitterion Form.
A zwitterion is a molecule that contains both positive and negative charges. Methionine can form a zwitterion when the pH of its surrounding environment is between 5.7 and 9.Under these conditions, the amino group of methionine becomes protonated (NH 3+), while the carboxyl group becomes deprotonated (COO –). The resulting zwitterionic form of methionine can be represented as:
- The amino group gains a positive charge.
- The carboxyl group loses a negative charge.
- The overall charge of the molecule becomes zero.
Factors Affecting Zwitterion Formation, Modify methionine to show its zwitterion form.
The pH dependence of zwitterion formation in methionine is due to the fact that the protonation and deprotonation of the amino and carboxyl groups are pH-dependent. At pH values below 5.7, the amino group is not protonated and the carboxyl group is protonated, resulting in a positively charged molecule.
At pH values above 9.2, the amino group is protonated and the carboxyl group is not protonated, resulting in a negatively charged molecule.Temperature and solvent polarity can also affect zwitterion formation. Higher temperatures favor the formation of zwitterions, while lower temperatures favor the formation of non-zwitterionic forms.
More polar solvents also favor the formation of zwitterions, while less polar solvents favor the formation of non-zwitterionic forms.For example, in water, which is a polar solvent, methionine exists primarily as a zwitterion at neutral pH. In non-polar solvents, such as hexane, methionine exists primarily as a non-zwitterionic form.
Applications of Methionine Zwitterion
The zwitterionic properties of methionine contribute to its biological significance and its use in pharmaceutical and industrial applications.In biological systems, the zwitterionic form of methionine is important for protein folding and stability. The positive charge of the amino group and the negative charge of the carboxyl group allow methionine to form electrostatic interactions with other amino acids, which helps to stabilize the protein structure.In
pharmaceutical applications, the zwitterionic properties of methionine are used to improve the solubility and bioavailability of drugs. By incorporating methionine into drug molecules, drug developers can increase the solubility of the drug in water, which makes it easier for the drug to be absorbed by the body.In
industrial applications, the zwitterionic properties of methionine are used to create surfactants and emulsifiers. Surfactants are used to reduce the surface tension of liquids, while emulsifiers are used to stabilize emulsions. The zwitterionic properties of methionine allow these compounds to interact with both water and oil, which makes them effective for use in a variety of industrial applications.
Quick FAQs
What is the structural formula of methionine?
CH3SCH2CH2CH(NH2)COOH
How does methionine form a zwitterion?
Under acidic conditions, the amino group becomes protonated, and under basic conditions, the carboxylic acid group becomes deprotonated, resulting in a net zero charge.
What factors influence zwitterion formation in methionine?
pH, temperature, and solvent polarity